Please sign up to our news alerts. Once you’ve submitted your request, you will receive your activation link by email.
Cosette Pharmaceuticals Appoints Vincent Colicchio as Senior Vice President of Operations
Cosette Pharmaceuticals, Inc., a leading specialty pharmaceuticals company, today announced the appointment of Vincent (Vin) Colicchio as Senior Vice President of Operations, effective February 3, 2025. In this role, Vin will lead Cosette’s manufacturing, global supply chain, and operational strategy, ensuring efficiency, and supply continuity as the company continues its strong trajectory of growth and transformation.
Cosette Pharmaceuticals Appoints Brad Leonard as Vice President, Commercial Operations
Cosette Pharmaceuticals, Inc., a leading specialty pharmaceuticals company, today announced the appointment of Brad Leonard as Vice President, Commercial Operations. In this role, Brad will spearhead Cosette’s generics business, including Generics Sales, Marketing/Pricing, Customer Service, and Demand Planning, as the company continues its expansion in these markets.
Avista Capital Partners and Hamilton Lane Announce Partnership in Cosette Pharmaceuticals
Avista Capital Partners (“Avista”), a leading New York-based private equity firm focused exclusively on healthcare, and Hamilton Lane, a leading global private markets investment management firm (Nasdaq: HLNE), today announced that funds managed by Hamilton Lane have acquired a significant equity interest in Cosette Pharmaceuticals (“Cosette”), a US-based specialty pharmaceutical company, from Avista and its co-investors.
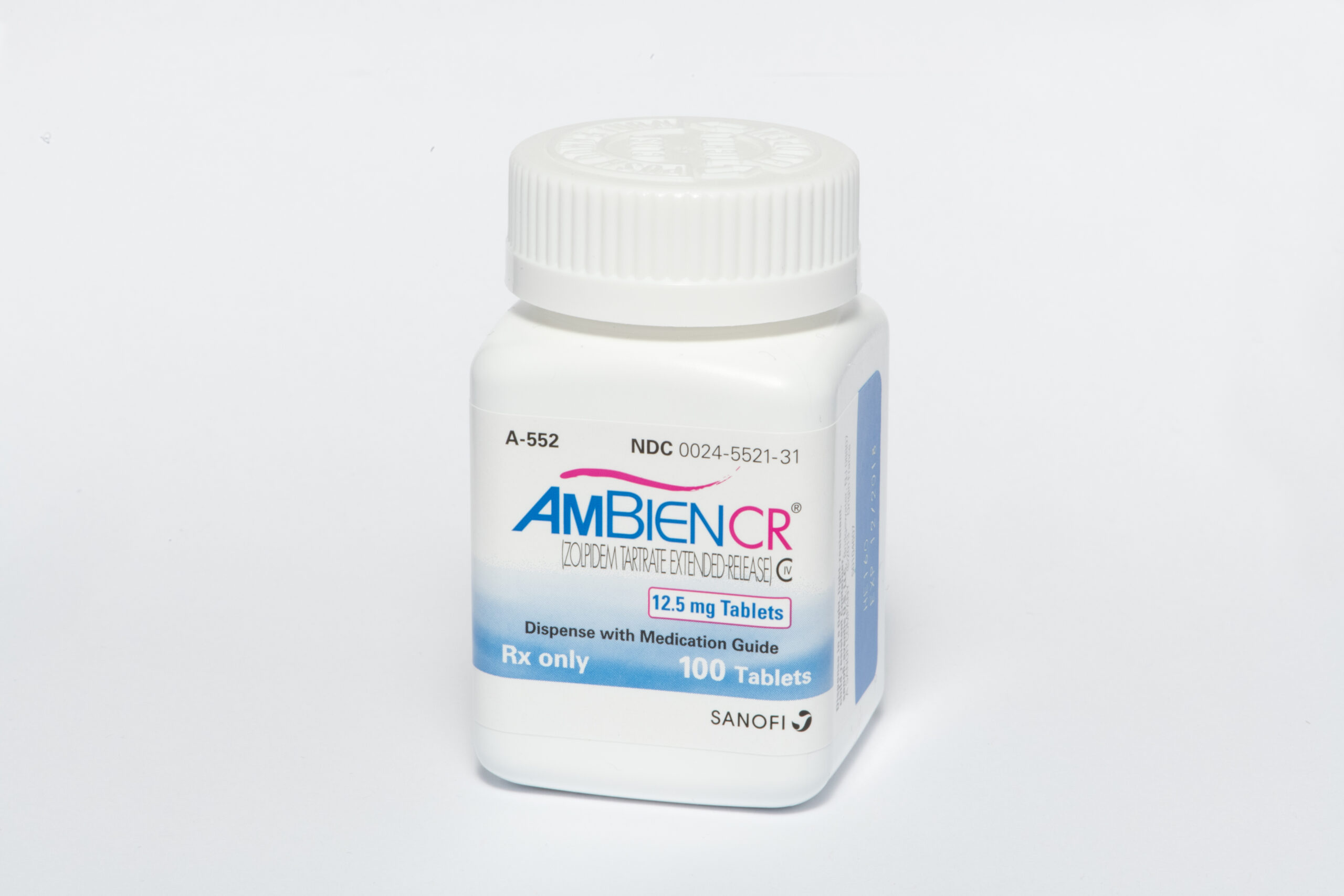
Cosette Pharmaceuticals Acquires Ambien® and Ambien CR® (Zolpidem Tartrate) Tabs from Sanofi US in the US Market
Cosette Pharmaceuticals, Inc. (“Cosette”), a US-based specialty pharmaceutical company, has completed the acquisition of Ambien® and Ambien CR® in the United States from Sanofi US.
Cosette Pharmaceuticals Announces the Approval and Launch of First Generic Version of RECTIV® (nitroglycerin) ointment, 0.4%, with 180 days Competitive Generic Therapy (CGT) exclusivity
Cosette Pharmaceuticals, Inc. ("Cosette") announced that the U.S. Food and Drug Administration (FDA) has approved the Abbreviated New Drug Applications (ANDA) for the first generic version of RECTIV® (nitroglycerin) ointment, 0.4%, with 180 days Competitive Generic Therapy (CGT) exclusivity. Cosette will imminently commence commercial shipments, triggering the 180 days exclusivity.
Cosette Pharmaceuticals Acquires Vyleesi® (Bremelanotide Injection) from Palatin Technologies Inc.
We are excited to announce that our women’s healthcare portfolio has expanded with the addition of Vyleesi® (Bremelanotide Injection) from Palatin Technologies! Vyleesi® is the only FDA-Approved as-needed treatment for the approximately 6 million premenopausal women who suffer from Hypoactive Sexual Desire Disorder (HSDD). This adds another commercial stage, patent protected product to Cosette’s rapidly expanding Women’s health portfolio.
Cosette Pharmaceuticals Appoints Ted Smolenski as Vice President of Portfolio & Business Development
Cosette Pharmaceuticals, Inc., a New Jersey-based specialty pharmaceutical company, today announced the appointment of Ted Smolenski as Vice President, Portfolio and Business Development, bolstering its product portfolio and business development team.
Apurva Saraf, President and CEO of Cosette Pharmaceuticals, Honored with “CEO of the Year” Award by Avista Capital Partners
We’re thrilled to announce that Apurva Saraf, our esteemed President and CEO, has been recognized with the esteemed “CEO of the Year” award by Avista Capital Partners. This remarkable achievement stands as a testament to Apurva’s exceptional leadership and the steadfast dedication of our team at Cosette Pharmaceuticals, Inc. towards driving growth and innovation.
Cosette Pharmaceuticals Partners With Mark Cuban Cost Plus Drug Company to Make CLOMID® More Affordable
Cosette Pharma announced today that it has entered into an agreement with Mark Cuban Cost Plus Drug Company, PBC (Cost Plus Drugs) to offer CLOMID® (clomiPHENE citrate) 50mg tablets at a low cash price via the Cost Plus Drugs online pharmacy.
Cosette Pharmaceuticals Launches Metronidazole Vaginal Gel 0.75%, AB-rated to MetroGel-Vaginal® 0.75%
Cosette Pharmaceuticals, Inc. announced today that the U.S. Food and Drug Administration (FDA) has approved its abbreviated new drug application (ANDA) for a generic version of MetroGel-Vaginal® (metronidazole vaginal gel 0.75%) by Bausch Health US, LLC.