Cosette Pharmaceuticals Appoints Vincent Colicchio as Senior Vice President of Operations
Cosette Pharmaceuticals, Inc., a leading specialty pharmaceuticals company, today announced the appointment of Vincent (Vin) Colicchio as Senior Vice President of Operations, effective February 3, 2025. In this role, Vin will lead Cosette’s manufacturing, global supply chain, and operational strategy, ensuring efficiency, and supply continuity as the company continues its strong trajectory of growth and transformation.
Cosette Pharmaceuticals Appoints Brad Leonard as Vice President, Commercial Operations
Cosette Pharmaceuticals, Inc., a leading specialty pharmaceuticals company, today announced the appointment of Brad Leonard as Vice President, Commercial Operations. In this role, Brad will spearhead Cosette’s generics business, including Generics Sales, Marketing/Pricing, Customer Service, and Demand Planning, as the company continues its expansion in these markets.
Avista Capital Partners and Hamilton Lane Announce Partnership in Cosette Pharmaceuticals
Avista Capital Partners (“Avista”), a leading New York-based private equity firm focused exclusively on healthcare, and Hamilton Lane, a leading global private markets investment management firm (Nasdaq: HLNE), today announced that funds managed by Hamilton Lane have acquired a significant equity interest in Cosette Pharmaceuticals (“Cosette”), a US-based specialty pharmaceutical company, from Avista and its co-investors.
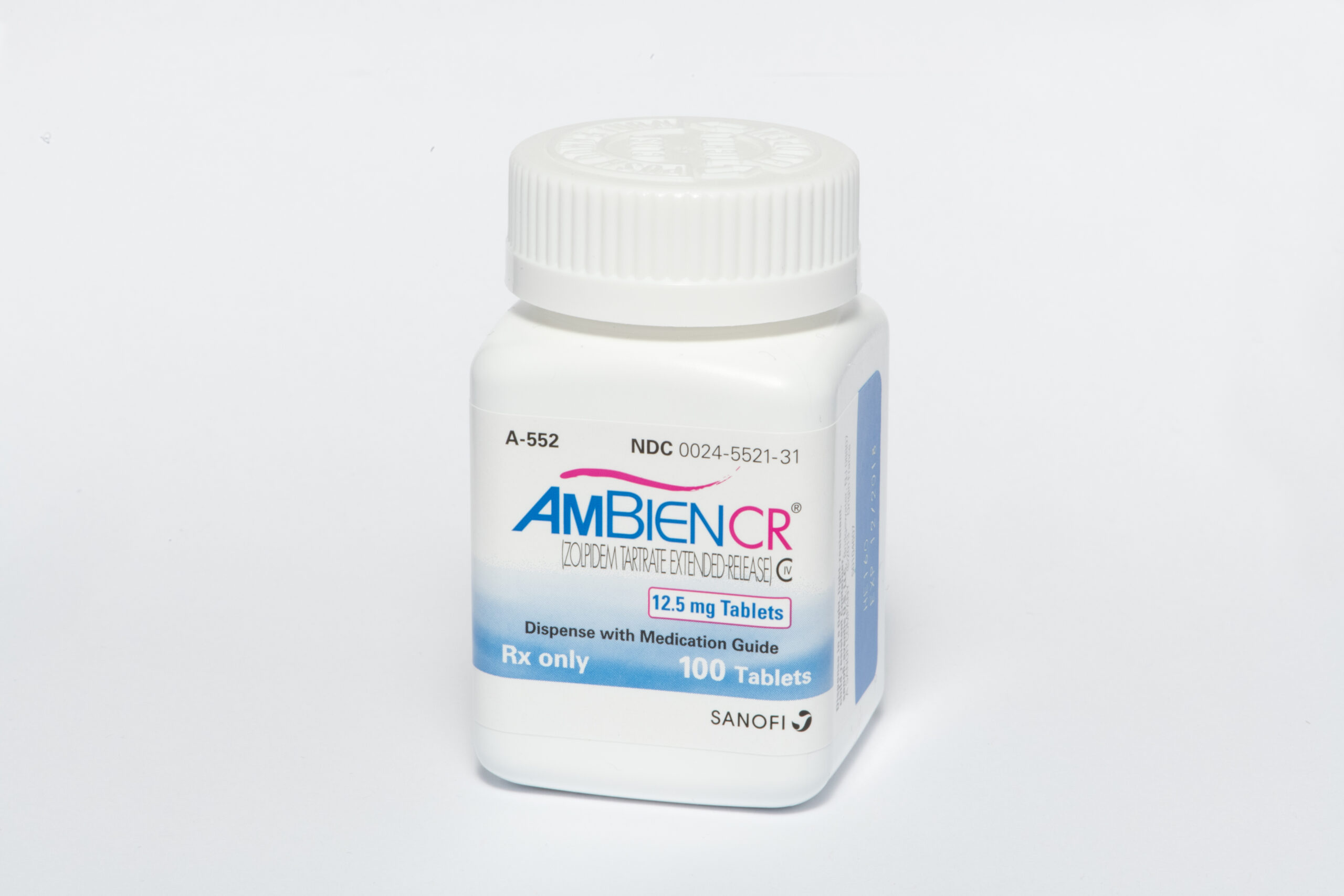
Cosette Pharmaceuticals Acquires Ambien® and Ambien CR® (Zolpidem Tartrate) Tabs from Sanofi US in the US Market
Cosette Pharmaceuticals, Inc. (“Cosette”), a US-based specialty pharmaceutical company, has completed the acquisition of Ambien® and Ambien CR® in the United States from Sanofi US.
Cosette Pharmaceuticals Announces the Approval and Launch of First Generic Version of RECTIV® (nitroglycerin) ointment, 0.4%, with 180 days Competitive Generic Therapy (CGT) exclusivity
Cosette Pharmaceuticals, Inc. ("Cosette") announced that the U.S. Food and Drug Administration (FDA) has approved the Abbreviated New Drug Applications (ANDA) for the first generic version of RECTIV® (nitroglycerin) ointment, 0.4%, with 180 days Competitive Generic Therapy (CGT) exclusivity. Cosette will imminently commence commercial shipments, triggering the 180 days exclusivity.
Cosette Pharmaceuticals Acquires Vyleesi® (Bremelanotide Injection) from Palatin Technologies Inc.
We are excited to announce that our women’s healthcare portfolio has expanded with the addition of Vyleesi® (Bremelanotide Injection) from Palatin Technologies! Vyleesi® is the only FDA-Approved as-needed treatment for the approximately 6 million premenopausal women who suffer from Hypoactive Sexual Desire Disorder (HSDD). This adds another commercial stage, patent protected product to Cosette’s rapidly expanding Women’s health portfolio.
Cosette Pharmaceuticals Appoints Ted Smolenski as Vice President of Portfolio & Business Development
Cosette Pharmaceuticals, Inc., a New Jersey-based specialty pharmaceutical company, today announced the appointment of Ted Smolenski as Vice President, Portfolio and Business Development, bolstering its product portfolio and business development team.
Apurva Saraf, President and CEO of Cosette Pharmaceuticals, Honored with “CEO of the Year” Award by Avista Capital Partners
We’re thrilled to announce that Apurva Saraf, our esteemed President and CEO, has been recognized with the esteemed “CEO of the Year” award by Avista Capital Partners. This remarkable achievement stands as a testament to Apurva’s exceptional leadership and the steadfast dedication of our team at Cosette Pharmaceuticals, Inc. towards driving growth and innovation.
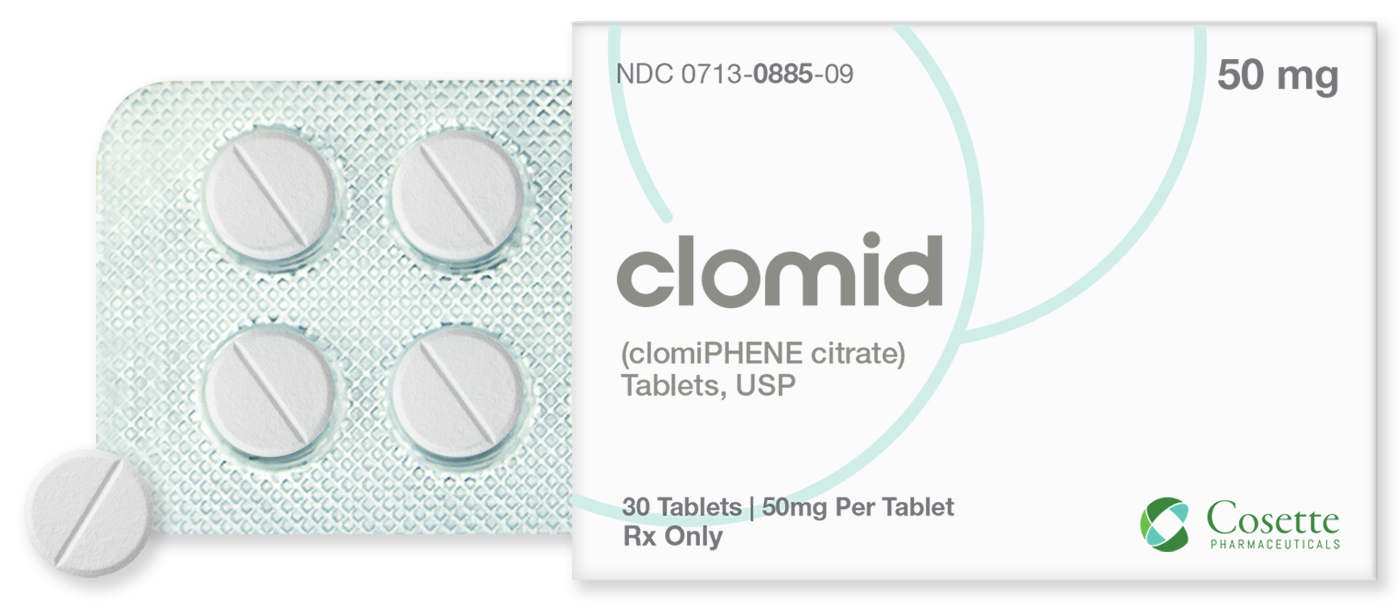
Cosette Pharmaceuticals Partners With Mark Cuban Cost Plus Drug Company to Make CLOMID® More Affordable
Cosette Pharma announced today that it has entered into an agreement with Mark Cuban Cost Plus Drug Company, PBC (Cost Plus Drugs) to offer CLOMID® (clomiPHENE citrate) 50mg tablets at a low cash price via the Cost Plus Drugs online pharmacy.
Cosette Pharmaceuticals Launches Metronidazole Vaginal Gel 0.75%, AB-rated to MetroGel-Vaginal® 0.75%
Cosette Pharmaceuticals, Inc. announced today that the U.S. Food and Drug Administration (FDA) has approved its abbreviated new drug application (ANDA) for a generic version of MetroGel-Vaginal® (metronidazole vaginal gel 0.75%) by Bausch Health US, LLC.
EY Announces Apurva Saraf, President and CEO of Cosette Pharmaceuticals as an Entrepreneur Of The Year® 2023 New Jersey Award Winner
Ernst & Young LLP (EY US) today announced that Apurva Saraf, President and CEO of Cosette Pharmaceuticals was named an Entrepreneur Of The Year® 2023 New Jersey Award winner. The Entrepreneur Of The Year® Awards program is one of the preeminent competitive awards for entrepreneurs and leaders of high-growth companies.
Cosette Pharmaceuticals Acquires Intrarosa® from Endoceutics, Inc.
Acquisition accelerates Cosette’s women’s health portfolio; with a differentiated, commercial stage, patent protected product.
Jackie Beltrani Joins Cosette Pharmaceuticals as Director, Commercial Operations.
We are pleased to announce that Jackie Beltrani has joined Cosette Pharmaceuticals as Director, Commercial Operations. Jackie will report to Kian Kazemi and will be based in the Bridgewater office.
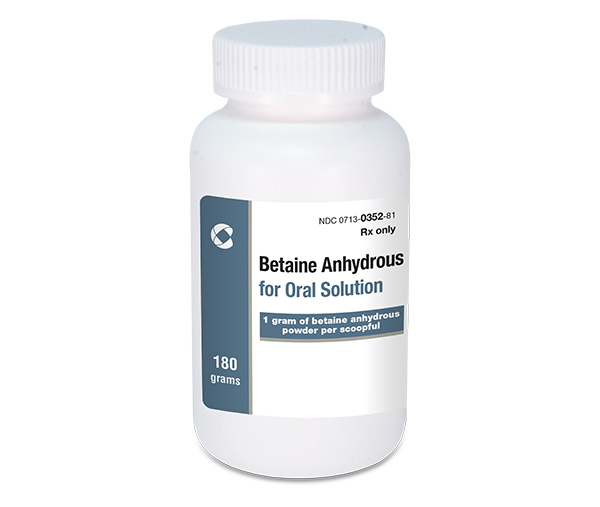
Cosette Pharmaceuticals Launches Betaine Anhydrous Powder, AB-rated to Cystadane® (betaine anhydrous for oral solution)
Apurva Saraf, President and CEO of Cosette, stated "This launch is further evidence of Cosette’s ability to partner globally and bring important drugs to the market, and furthers our transformation into a specialty pharmaceutical company. We stay true to our firm belief of Innovating Every Day, to be a trusted partner of choice, and to continue the important work of serving U.S. patients and prescribers.”
Cosette Pharmaceuticals Announces the Approval and Launch of Metronidazole Gel USP, 1%.
Cosette Pharmaceuticals, Inc ("Cosette") announced that the U.S. Food and Drug Administration (FDA) has approved the Abbreviated New Drug Applications (ANDA) for Metronidazole Gel USP, 1%.
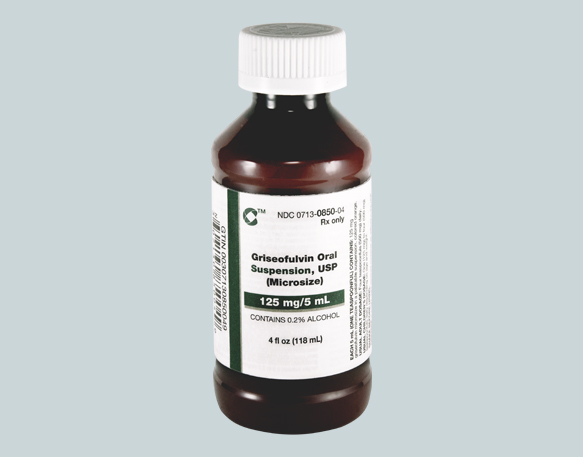
Cosette Pharmaceuticals Announces the launch of Griseofulvin Oral Suspension, 125 mg/5 mL.
Cosette Pharmaceuticals, Inc began shipping Griseofulvin Oral Suspension, 125 mg/5 mL (0713-0850-04) into all channels in the United States.
Cosette Pharmaceuticals Appoints Kevin Hickey, Vice President, Brand Sales and Marketing to Accelerate Transformation
“We are very excited to have Kevin Hickey join our team. His tremendous experience in strategy and execution of branded products will be instrumental as we integrate our eight cardio branded products through our transformative acquisition from Daiichi Sankyo and get ready for the imminent launches of several new products and lifecycle management strategies.” said Apurva Saraf, President and CEO, Cosette Pharmaceuticals.
Cosette Pharmaceuticals Announces the Approval and Launch of First Generic Versions of TAZORAC® (tazarotene) gel, 0.05% and 0.1%, with 180 days Competitive Generic Therapy (CGT) exclusivity
Cosette announced that the U.S. Food and Drug Administration (FDA) has approved the Abbreviated New Drug Applications (ANDA) for the first generic versions of TAZORAC® (tazarotene) gel, 0.05% and 0.1%, with 180 days Competitive Generic Therapy (CGT) exclusivity. Cosette has already commenced commercial shipments, triggering the 180 days exclusivity.
Ganesh Pillarisetti has joined Cosette Pharmaceuticals as Vice President, IT
Ganesh Pillarisetti has joined the executive team as Vice President, IT at Cosette Pharmaceuticals. Ganesh will report to our President and CEO, Apurva Saraf, and will be based out of our office at South Plainfield, New Jersey. In this critical role, Ganesh's expertise in leveraging IT to streamline processes to increase both efficacy and efficiencies in our core areas of manufacturing, supply chain, quality, finance etc. will help us strongly deliver on our growth strategy of inorganic and organic growth through relentless execution.
Cosette Pharmaceuticals Appoints Rick Casten as CFO Amid Transformational Growth
Bridgewater, NJ. July 6, 2022. Cosette Pharmaceuticals, Inc., a New Jersey-based specialty pharmaceutical company, today announced the appointment of Rick Casten as Chief Financial Officer (CFO), bolstering its executive leadership team.
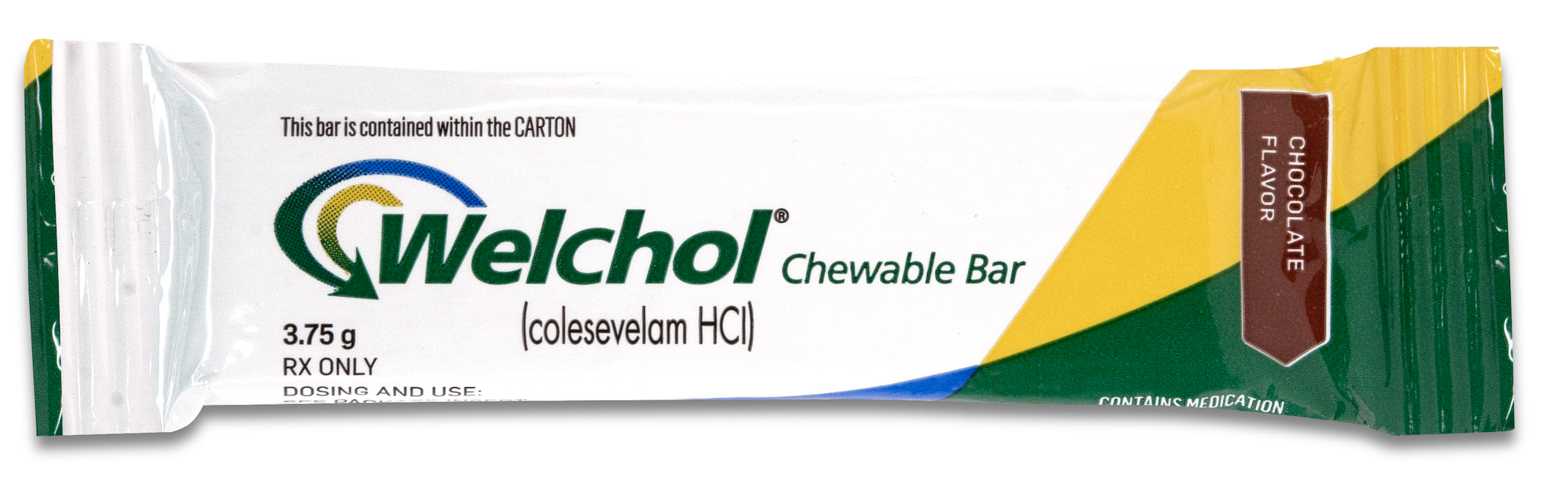
Cosette Pharmaceuticals Receives U.S. Patent (US 11,291,628) For Welchol® Chewable Bar, A Novel Oral Drug Delivery System to Deliver Colesevelam Hcl
BRIDGEWATER, NJ – JUNE 7, 2022: Cosette Pharmaceuticals, Inc., a New Jersey-based specialty pharmaceutical company, announced today that the U.S. Patent and Trademark Office (USPTO) issued U.S. Patent No. 11,291,628 for Welchol® Chewable Bar, the Company’s novel drug delivery system of Colesevelam that is easy to handle and ingest, which provides patent protection until 2037.
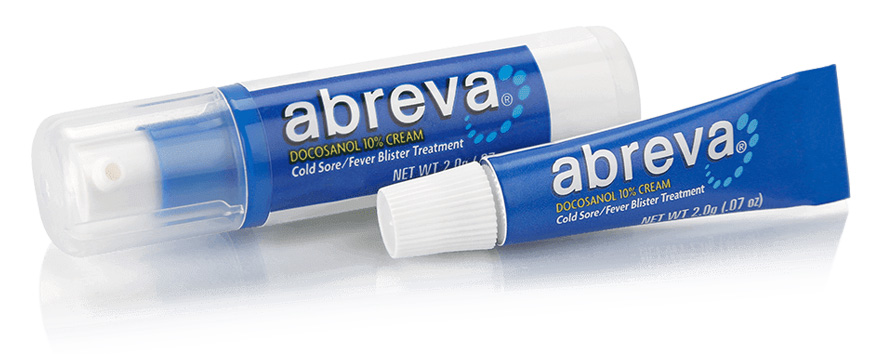
Cosette Pharmaceuticals and Alembic Pharmaceuticals Limited Announce the Approval and Launch of a National Brand Equivalent (NBE) of Abreva® (Docosanol Cream 10%) Including the First Approval in the Pump Format
Bridgewater, NJ and Vadodara, India May 24, 2022/PRNewswire/Cosette Pharmaceuticals, Inc ("Cosette"), in collaboration with Aleor Dermaceuticals, a wholly owned subsidiary of Alembic Pharmaceuticals (“Alembic”; BSE: 533573), announced that the U.S. Food and Drug Administration (FDA) has approved the Abbreviated New Drug Application (ANDA) for an NBE version of Abreva® (Docosanol cream 10%) in a 2 gm tube and pump formats. Shipments of the product will begin imminently.
Cosette Pharmaceuticals Launches First Injectable Product – Prochlorperazine Edisylate Injection in the U.S.
Cosette Pharmaceuticals, Inc., a New Jersey-based pharmaceutical company, today announced the launch of Prochlorperazine Edisylate injection, 10 mg/2ml (5mg/ml).
Cosette Pharmaceuticals Acquires Rights to Eight Branded Products from Daiichi Sankyo
Cosette Pharmaceuticals, Inc., a New Jersey-based specialty pharmaceutical company, announced today that it has closed a transaction to acquire the US sales and distribution rights to eight branded commercial products from leading Japanese pharmaceutical company, Daiichi Sankyo Company, Ltd (4568:JP) and affiliates.
Mindy Hanna has joined Cosette Pharmaceuticals as Senior Director, Human Resources
We are pleased to announce Mindy Hanna has joined Cosette Pharmaceuticals as Senior Director, Human Resources. Mindy will report to Apurva Saraf and will be based in the South Plainfield office.
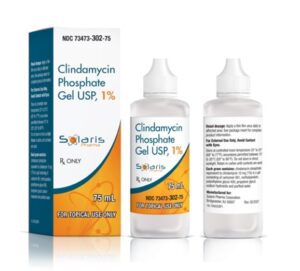
Solaris Pharma Receives Approval for First Generic Version of Clindagel® (clindamycin phosphate EQ 1%)
Solaris Pharma Corporation (“Solaris” or the “Company”) announced today that the Company has received an abbreviated new drug application (“ANDA”) approval from the U.S. Food and Drug Administration (“FDA” or “Agency”) for the first generic version of Clindagel® (clindamycin phosphate EQ 1%). This product will be manufactured in the United States by Cosette, Solaris’ manufacturing partner. The product will be launched immediately.
Cosette Pharmaceuticals, Inc. Announces FDA Approval of ANDA for Hydrocortisone Valerate Cream USP, 0.2%
Cosette Pharmaceuticals, Inc., a New Jersey-based specialty generic pharmaceutical company, has received a first cycle approval of the Company’s abbreviated new drug application (ANDA) from the U.S. Food and Drug Administration (FDA) of Hydrocortisone Valerate Cream USP, 0.2%. This is Cosette’s first approval for 2021 from its internally-developed pipeline of prescription pharmaceutical products.
Cosette Pharmaceuticals Appoints Seasoned Industry Executive Apurva Saraf as President and Chief Executive Officer
We are very pleased to welcome Apurva to the Cosette team. His deep knowledge of the pharmaceutical industry, along with his corporate strategy and business development experience, will be of great value as Cosette continues to expand its portfolio of differentiated complex products, both internally and through strategic collaborations,” stated Sriram Venkataraman, Partner of Avista Capital Partners.