Please sign up to our news alerts. Once you’ve submitted your request, you will receive your activation link by email.
EY Announces Apurva Saraf, President and CEO of Cosette Pharmaceuticals as an Entrepreneur Of The Year® 2023 New Jersey Award Winner
Ernst & Young LLP (EY US) today announced that Apurva Saraf, President and CEO of Cosette Pharmaceuticals was named an Entrepreneur Of The Year® 2023 New Jersey Award winner. The Entrepreneur Of The Year® Awards program is one of the preeminent competitive awards for entrepreneurs and leaders of high-growth companies.
Cosette Pharmaceuticals Acquires Intrarosa® from Endoceutics, Inc.
Acquisition accelerates Cosette’s women’s health portfolio; with a differentiated, commercial stage, patent protected product.
Jackie Beltrani Joins Cosette Pharmaceuticals as Director, Commercial Operations.
We are pleased to announce that Jackie Beltrani has joined Cosette Pharmaceuticals as Director, Commercial Operations. Jackie will report to Kian Kazemi and will be based in the Bridgewater office.
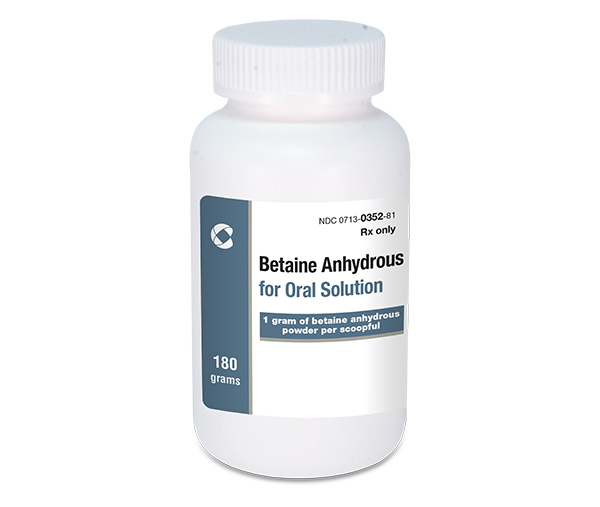
Cosette Pharmaceuticals Launches Betaine Anhydrous Powder, AB-rated to Cystadane® (betaine anhydrous for oral solution)
Apurva Saraf, President and CEO of Cosette, stated "This launch is further evidence of Cosette’s ability to partner globally and bring important drugs to the market, and furthers our transformation into a specialty pharmaceutical company. We stay true to our firm belief of Innovating Every Day, to be a trusted partner of choice, and to continue the important work of serving U.S. patients and prescribers.”
Cosette Pharmaceuticals Announces the Approval and Launch of Metronidazole Gel USP, 1%.
Cosette Pharmaceuticals, Inc ("Cosette") announced that the U.S. Food and Drug Administration (FDA) has approved the Abbreviated New Drug Applications (ANDA) for Metronidazole Gel USP, 1%.
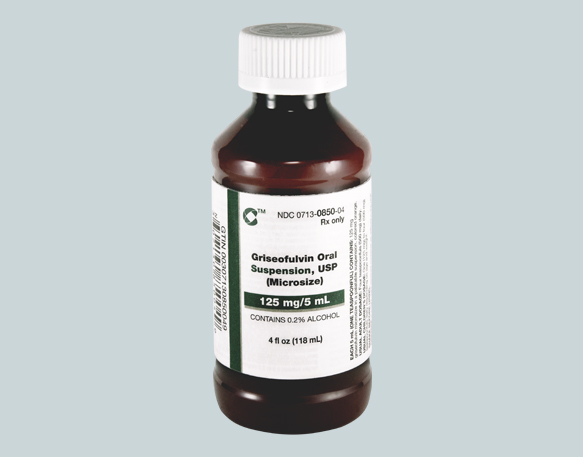
Cosette Pharmaceuticals Announces the launch of Griseofulvin Oral Suspension, 125 mg/5 mL.
Cosette Pharmaceuticals, Inc began shipping Griseofulvin Oral Suspension, 125 mg/5 mL (0713-0850-04) into all channels in the United States.
Cosette Pharmaceuticals Appoints Kevin Hickey, Vice President, Brand Sales and Marketing to Accelerate Transformation
“We are very excited to have Kevin Hickey join our team. His tremendous experience in strategy and execution of branded products will be instrumental as we integrate our eight cardio branded products through our transformative acquisition from Daiichi Sankyo and get ready for the imminent launches of several new products and lifecycle management strategies.” said Apurva Saraf, President and CEO, Cosette Pharmaceuticals.
Cosette Pharmaceuticals Announces the Approval and Launch of First Generic Versions of TAZORAC® (tazarotene) gel, 0.05% and 0.1%, with 180 days Competitive Generic Therapy (CGT) exclusivity
Cosette announced that the U.S. Food and Drug Administration (FDA) has approved the Abbreviated New Drug Applications (ANDA) for the first generic versions of TAZORAC® (tazarotene) gel, 0.05% and 0.1%, with 180 days Competitive Generic Therapy (CGT) exclusivity. Cosette has already commenced commercial shipments, triggering the 180 days exclusivity.